Iron in the body is a chemical formula. Iron: the structure of the atom, distribution in nature. Physical and chemical properties of iron
- Position in PS
- Features of the structure of the atom
- Being in nature
- Value for plants and animals
- Physical properties
- Iron species
- Options for obtaining
- Features of chemical properties
- Areas of use
- Story
- Properties
- Using
- Iron as a chemical component of the body
- Iron as chemical element
- Iron corrosion
Iron is a metal element of a secondary subgroup of VIIl of group 4 of the periodical system of chemical elements. He is a representative of d-elements. At the outer energy level of the iron atom are two s-electrons.
And on the front-level energy level, the d-sublevel is filled. In its compounds, Ferum is able to exhibit +2 and +3 oxidation states.
Mass fraction of iron in the earth's crust is 5%. It ranks fourth in prevalence in nature. The most important iron ores are: magnetite Fe3O4, hematite Fe2O3, limonite - Fe2O3 nH2O, siderite FеCO3, pyrite FeS2.
Iron is a biologically important element. It is contained in the organisms of all animals and in plants. Iron is part of the cytoplasm of plants, is involved in the process of photosynthesis. An adult’s body contains about 4 grams of iron. It accumulates mainly in the liver, bone marrow, spleen. But the main part of iron is part of hemoglobin - red blood pigment, which performs the function of transporting oxygen from the lungs to the tissues, and in the opposite direction - carbon dioxide. A lack of iron leads to a dangerous disease - anemia. Therefore, be sure to use food products , rich Ferum: parsley, liver, veal, buckwheat, dried apricots and others.
Is pure iron a silvery white shiny metal with a melting point of 1535? With a density of 7.87 g / cm3. Iron differs from other metals in magnetic properties. Iron is a very ductile metal and is easy to process.
Iron is one of the metals of average activity. Chemically pure iron is resistant to corrosion. However, insignificant fractions of impurities deprive it of this property.
Iron burns in oxygen. The product of this reaction is Iron Ogarina:
3Fe + 2O2 = Fe3O4.
Iron reacts with other non-metals - halogens, sulfur, carbon. When interacting with strong oxidizing agents, for example, chlorine, iron compounds are formed with an oxidation state of +3:
2Fe + 3Cl2 = 2FeCl3,
and when interacting with less active oxidizing agents, compounds with the degree of iron oxidation +2 are formed:
With high temperature iron reacts with water vapor. As a result of the reaction, iron ozarin and hydrogen are formed:
3Fe + 4H2O Fe3O4 + 4H2?
Iron reacts with acid solutions to form salts and release gaseous hydrogen:
Fe + H2SO4 = FeSO4 + H2?
Under the action of concentrated sulfuric and nitric acids, a dense oxide film forms on the surface of iron, for these concentrated acids can be stored and transported in steel tanks.
Iron replaces less active metals when interacting with solutions of their salts:
Fe + CuSO4 = FeSO4 + Cu.
Iron is corroded in humid air. Its product is rust. Due to its porosity, rust does not prevent the access of oxygen and moisture to the metal, which leads to further destruction of the metal.
Iron is the most important metal of modern technology. In its pure form, iron is almost never used, but about 90% of the metals used by mankind are iron-based alloys. There is a lot of iron smelted in the world, about 50 times more than aluminum, not to mention other metals. Iron-based alloys are created that are able to withstand high and low temperatures, vacuum and high pressures , aggressive media, etc .. Iron alloys are widely used as structural and artistic materials.
Consider the electronic structure of the iron atom, as well as its location in the periodic table. Identify the basic physical and Chemical properties this element, the area of use.
Position in PS
Iron is the d-element of group 8 (a subgroup). It has 26 serial number, relative atomic mass - 56, in its atom contains 26 protons, 26 electrons, and also 30 neutrons. This metal has an average chemical activity, shows reducing properties . Typical oxidation states: +2, +3.
Features of the structure of the atom
What is the electronic gland? If we consider the distribution of electrons in energy levels, we obtain the following option:
2e; 8e; 14 th; 2nd. Such a structure of the electron shell of an atom of iron indicates its location in a secondary subgroup, confirms belonging to the g-family of elements.

Being in nature
Iron is one of the most common chemical elements in nature. In the crust, its percentage is about 5.1%. In greater quantities in the depths of our planet there are only three elements: silicon, aluminum, oxygen.
Iron ores are found in different regions of the earth. Alchemists discovered compounds of this metal in soils. In the production of iron, ores are chosen in which its content exceeds 30 percent.
The magnetic iron ore contains about seventy two percent of the metal. The main magnetite deposits are located in the Kursk Magnetic Anomaly, as well as in the Southern Urals. In krovavike, the percentage of iron reaches 65 percent. Hematite was found in the Krivoy Rog region.

Value for plants and animals
What role does iron play in living organisms? The structure of the atom explains its reducing properties. This chemical element is part of hemoglobin, giving it a characteristic red color. About three grams of pure iron, most of which is included in hemoglobin, is found in the body of an adult. The main purpose is to transfer to the tissues from the lungs of active oxygen, as well as the output of the resulting carbon dioxide.
Needed this metal and plants. Being a part of the cytoplasm, he takes an active part in the processes of photosynthesis. If there is not enough iron in the plant, its leaves are white in color. With minimal fertilizing with iron salts, the leaves of the plants become green.
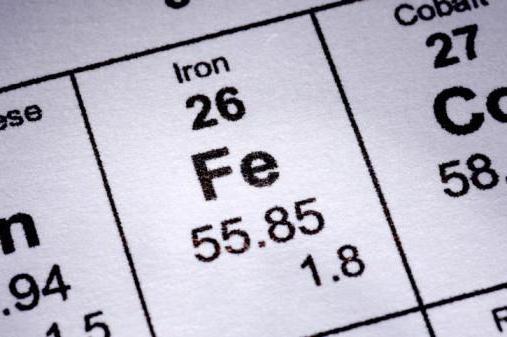
Physical properties
We considered the structure of the iron atom. The scheme confirms the presence of a metallic luster for this element (there are valence electrons). The silver-white metal has a rather high melting point (1539 degrees Celsius). Due to good ductility, this metal is easy to rolling, stamping, forging.
The ability to magnetize and demagnetize, characteristic of iron, has made it an excellent material for the production of cores of powerful electromagnets in various apparatuses and electrical machines.
How active is iron? The structure of the atom shows the presence on the outer level of two electrons that will be given away during a chemical reaction. To increase its hardness and strength, additional rolling and hardening of the metal are carried out. Such processes are not accompanied by a change in the structure of the atom.

Iron species
The electronic structure of the iron atom, the scheme of which was considered above, explains its chemical characteristics. In technically pure metal, which is a low carbon steel, the main component is iron. About 0.04 percent of carbon was detected as impurities, and phosphorus, nitrogen, and sulfur are also present.
Chemically pure iron in its external parameters is similar to platinum. It has a high resistance to corrosion processes, resistant to acids. With the slightest introduction of impurities into the pure metal, its unique characteristics disappear.
Options for obtaining
The structure of the atoms of aluminum and iron indicate that amphoteric aluminum belongs to the main subgroup, the possibility of using it in the process of extracting iron from its oxides. Alumothermia, carried out at elevated temperatures, allows to produce pure metal from natural ores. In addition to aluminum, carbon (2) and coal are chosen as strong reducing agents.
Features of chemical properties
What chemical properties does iron have? The structure of the atom explains its reducing activity. Iron is characterized by the formation of two rows of compounds having oxidation states of +2, +3.
In humid air, the process of rusting (corrosion) of the metal occurs, as a result of which iron hydroxide (3) is formed. With oxygen, the heated iron wire reacts with the appearance of black iron oxide powder (2,3), called iron oxide.
At high temperatures, the metal is able to interact with water vapor, thus forming a mixed oxide. The process is accompanied by the release of hydrogen.
The reaction with non-metals occurs only with the preliminary heating of the initial components.
Iron can be dissolved in dilute sulfuric or hydrochloric acids without preheating the mixture. Concentrated sulfuric and hydrochloric acids passivate this metal.
What other chemical properties does iron have? The structure of the atom of this element indicates its average activity. This is confirmed by the location of iron to hydrogen (H2) in a series of stresses. Consequently, it can displace all the metals from the salts, which are located to the right in the Beketov series. So, in the reaction with copper chloride (2), carried out when heated, the release of pure copper and obtaining a solution of iron chloride (2).
 Areas of use
Areas of use
Most of the iron is used in the production of iron and steel. In iron, the percentage of carbon is 3-4 percent, in steel - no more than 1.4 percent. This non-metal performs the function of an element that increases the strength of the connection. In addition, it has a positive effect on the corrosion properties of alloys, increases the resistance of the material to elevated temperature.
Vanadium additives are necessary to increase the mechanical strength of steel. Chromium increases the resistance to aggressive chemicals.
The ferromagnetic properties of this chemical element made it popular in industrial installations incorporating electromagnets. In addition, iron has found its use in the souvenir industry. On its basis, various souvenirs are made, for example, colorful fridge magnets.
Strength and malleability allow you to use metal to create armor, various types of weapons.
(3) used to purify water from impurities. In medicine, 26 elements of a periodic use in the treatment of diseases such as anemia. If there is a shortage of red blood cells fast fatiguability The skin gets an unnatural pale color. Iron preparations help to eliminate a similar problem, to return the body to full activity. Of particular importance is iron for the activity of the thyroid gland, the liver. To avoid serious problems in the human body, it’s enough to consume about 20 mg of this metal per day.
Here you can download a lesson on the topic: "Iron. The position of iron in the periodic table and the structure of its atom. Being in nature. Physical and chemical properties of iron" for the subject: Chemistry. This document will help you prepare good and quality material for the lesson.
Date _____________ Class _______________
Subject: Iron. The position of iron in the periodic system and the structure of its atom. Being in nature. Physical and chemical properties of iron .
Objectives: to consider the electronic structure of the iron atom; study its chemical and physical properties.
Working process
1. The organizational moment of the lesson.
2. The study of new material.
Iron - chemical element
1. The position of iron in the periodic table of chemical elements and the structure of its atom
Steel is used for the manufacture of machines, various building materials, beams, sheets, rolled products, rails, tools and many other products. For the production of various grades of steel, so-called alloying additives are used, which are used by various metals: Mn, Cr, Mo and others that improve the quality of steel.
3. Consolidation of the material studied
№1. Make the equations of the reaction of obtaining iron from its oxides Fe 2 O 3 and Fe 3 O 4, using as a reducing agent:
a) hydrogen;
b) aluminum;
c) carbon monoxide (II).
For each reaction, make an electronic balance.
№2. Carry out the transformation according to the scheme:
Fe 2 O 3 -> Fe - + H2O, t -> X - + CO, t -> Y - + HCl -> Z
What are the products X, Y, Z?
4. Homework.
P.43, Ex.1-3, tasks 1, 4 on p. 136
(the so-called meteoric iron, which contains more than 90% Fe). In compounds with oxygen and other elements it is widespread in the composition of many minerals and ores. According to the prevalence in the crust (5.00%) this is the third (after silicon and aluminum) element; it is believed that the earth’s core consists mainly of iron. The main minerals are hematite (iron red) Fe 2 O 3; limonite Fe 2 O 3 · nH 2 O (n = 1–4) contained, for example, in bog ore; magnetite (magnetic iron ore) Fe 3 O 4 and siderite FeCO 3. The most common iron mineral, which is not, however, the source of its production, is pyrite (sulfur pyrite, iron pyrite) FeS 2, which is sometimes called for its yellow glitter fool gold or cat gold, although in fact it often contains small amounts of copper, gold , cobalt and other metals.
PROPERTIES OF IRON Atomic number 26 Atomic mass 55.847 Isotopes: stable 54, 56, 57, 58 unstable 52, 53, 55, 59 Melting point, ° С 1535 Boiling point, ° С 3000 Density, g / cm3 7.87 Hardness (according to Moos ) 4.0-5.0 Content in the Earth's crust,% (mass.) 5.00 Oxidation degree: characteristic +2, +3 other values +1, +4, +6
Story
Iron (elemental) has been known and used since prehistoric times. The first iron products were probably made of meteoric iron in the form of amulets, jewels and working tools. About 3,500 years ago, man discovered a way to restore red earth containing iron oxide to metal. Since then, a huge number of various products have been made of iron. It played an important role in the development of the material culture of mankind. Nowadays, iron is mainly (95%) smelted from ores in the form of iron and steel, and in relatively small quantities is obtained by reduction of metallized pellets, and pure iron - by thermal decomposition of its compounds or by electrolysis of salts.
Properties
Metallic iron is a greyish-white shiny, hard plastic material. Iron crystallizes in three modifications (α, γ, δ). α-Fe has a body-centered cubic crystal lattice, it is chemically stable up to 910 ° С. At 910 ° C, α-Fe becomes γ-Fe, stable in the range of 910-1400 ° C; γ-Fe crystallizes in a face-centered cubic crystal lattice. At temperatures above 1400 ° C, δ-Fe is formed with a lattice substantially similar to the α-Fe lattice. Iron is a ferromagnet, it easily magnetizes, but loses its magnetic properties when the magnetic field is removed. With an increase in temperature, the magnetic properties of iron deteriorate and above 769 ° C it practically does not lend itself to magnetization (sometimes iron in the range of 769-910 ° C is called &-Fe); γ-Fe is not a magnetic material.
Using
Iron is one of the most serviceable metals in an alloy with carbon (steel, cast iron) - a high-strength basis of structural materials. As a material with magnetic properties, iron is used for the cores of electromagnets and anchors of electric machines, as well as layers and films on magnetic tapes. Pure iron - a catalyst in chemical processes, component medicines in medicine.
Iron as a chemical component of the body
Iron is an essential chemical component of many vertebrate, invertebrate, and some plant organisms. It is part of the heme (pigment of red blood cells - red blood cells) of hemoglobin of the blood, muscle tissue, bone marrow, liver and spleen. Each hemoglobin molecule contains 4 iron atoms that are capable of creating a reversible and fragile bond with oxygen, forming oxyhemoglobin. Blood containing oxyhemoglobin circulates through the body, supplying oxygen to the tissues for cellular respiration. Therefore, iron is necessary for respiration and the formation of red blood cells. Myoglobin (or muscular hemoglobin) supplies oxygen to muscles. The total amount of iron in the human body (average weight 70 kg) is 3-5 g. Of this amount, 65% of Fe is in hemoglobin. 10 to 20 mg of Fe is required daily to ensure the normal metabolism of the average adult. Red meat, eggs, yolk, carrots, fruits, any wheat and green vegetables mainly provide the body with iron with a normal diet; for anemia associated with a lack of iron in the body, take medications gland.
Iron as chemical element
From a chemical point of view, iron is a fairly active metal, exhibiting characteristic oxidation states of +2, +3, less often +1, +4, +6. It directly combines with some elements, with S it forms FeS - iron (III) sulfide, with halogens, except iodine, iron (III) halides, such as FeCl 3. It is easily oxidized; with oxygen gives oxides FeO, Fe 2 O 3, Fe 3 O 4 (FeO + Fe 2 O 3), easily corrodes (rust). Displaces hydrogen from water vapor at high temperatures. It is dissolved in dilute acids (for example, HCl, H 2 SO 4, HNO 3), displacing hydrogen and forming salts of Fe (II) (respectively, FeCl 2, FeSO 4, Fe (NO 3) 2). In moderately concentrated H 2 SO 4 and HNO 3, iron dissolves with the formation of Fe (III) salts, and in highly concentrated ones it is passivated and does not react. The passivity of iron is apparently due to the formation of an iron oxide film on its surface, which, however, is easily destroyed by simple scraping.
Iron corrosion
Iron rusting (atmospheric corrosion of iron) is the oxidation of iron by atmospheric oxygen. The reaction occurs in the presence of ions of salts dissolved in water, and ions formed during the dissociation of carbonic acid - the product of the interaction of atmospheric carbon dioxide and moisture. The result is loose red rust, or hydrated oxide of the composition Fe 2 O 3 · nH 2 O.
Connections
Complex compounds
Is pure iron a silvery white shiny metal with a melting point of 1535?How active is iron?
What other chemical properties does iron have?